Electron Transport Chain
The Cellular Power Plant: Mitochondria and the Electron Transport Chain
The majority of our ATP isn't produced in just any part of the cell. Instead, it's manufactured in specialized structures called mitochondria (shown in the pink shaded region of the image above) that is often referred to as the cell's power plants. These remarkable organelles have a unique structure perfectly designed for maximum energy production:
Mitochondrial Architecture
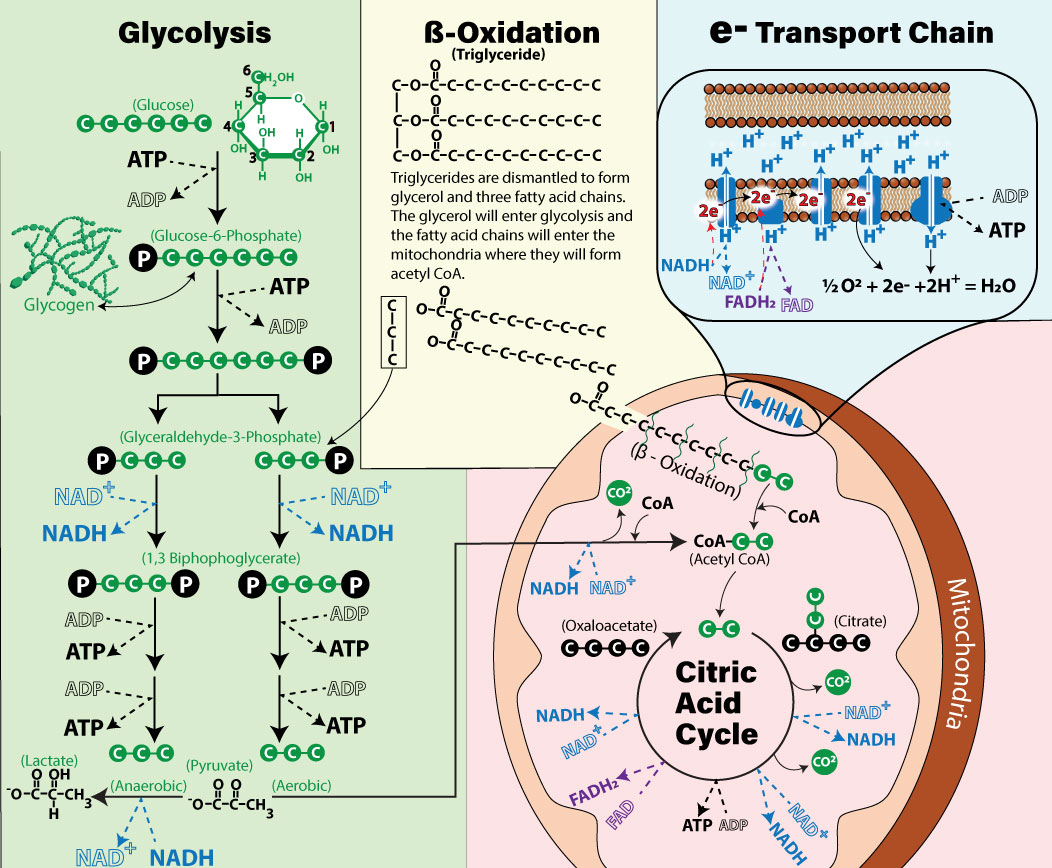
There are three important structures that will be used in energy production. Essentially a mitochondrion is like a mini cell, complete with its very own membrane, only its membrane is a double membrane structure. It has an outer membrane (controls what enters and exits) and an inner membrane (houses the electron transport chain which is shown in the light blue highlighted section of the picture above). The center of the mitochondrion is called the matrix, and this is where key reactions will occur in a process we call the Citric Acid Cycle. We plan to go into much more detail but here is a quick overview: The electron transport chain is located on the inner membrane and receives electrons from NADH and FADH2 which it will use to generate most of our ATP.
Let's return to our marathon runner. In their leg muscles:
- Thousands of mitochondria are working overtime
- Each mitochondrion's electron transport chain is running at full capacity
- This allows sustained energy production for the long race
The more mitochondria in muscle cells, the better the endurance This is why endurance athletes often have more mitochondria in their muscle cells than the average person. Their bodies have adapted to produce more cellular power plants to meet the high energy demands of their activities.
Electron Transport Chain (Light Blue Shaded Region in Image Above)
The term electron transport refers to the proteins on the inner membrane of the mitochondria that will take hydrogen atoms and electrons from NADH and FADH2 and then ultimately use the energy in the electrons to make ATP (the battery discussed previously). Recall that NAD+ and FAD picked up high energy electrons and hydrogens from C-H bonds. In the inner membrane of the mitochondrion is a series of protein complexes that will receive the electrons and pass them from one complex to another. NADH passes 2 high energy electrons onto a protein complex (Complex I) in the inner membrane of the mitochondria. This complex does two things. First, it accepts a pair of high energy electrons from NADH. Once transferred to Complex I, electrons move through an intricate maze within this protein complex. This flow of electrons creates an electrical charge imbalance, causing four protons (positively charged hydrogen ions; H+) to be pumped from the mitochondrial matrix into the intermembrane space. This process generates a proton gradient, a crucial step in cellular respiration. After completing their journey through Complex I, the electrons are transferred to Complex III via an electron shuttle called coenzyme Q (CoQ). In Complex III, the electrons pass through another maze-like pathway, powering the transport of four more protons from the matrix to the intermembrane space. However, as electrons move through the chain, they lose energy, much like a ball rolling downhill slows as it approaches the bottom. FADH₂, another electron carrier, contributes its electrons differently. Because its electrons carry less energy than those from NADH, they bypass Complex I and instead enter the chain at Complex II. From there, CoQ shuttles the electrons directly to Complex III, allowing them to continue their downward flow. After leaving Complex III, the electrons are handed off to another shuttle molecule, cytochrome C, which carries them to Complex IV. In Complex IV, the electrons have just enough energy left to pump two additional protons into the intermembrane space. At this stage, the electrons have reached the "bottom of the hill," with almost no energy remaining. To keep the electron transport chain functional, these low-energy electrons must be removed. Oxygen accepts the electrons from the last protein complex (complex IV) of the chain.
As oxygen accepts electrons, the oxygen becomes reactive and capable of forming a covalent bond with two protons and water is formed (H2O). If you have ever wondered what oxygen does and why it is so important to breathe into our bodies, now you know. Oxygen is the final electron acceptor. The important message in all of this is that electron energy is used to transport H+ ions to the intermembranous space and this sets up an electrochemical gradient that favors the movement of H+ ions back into the matrix. The H+ ions are very crowded in the intermembranous space, which makes the driving force to move out of the space very strong. The H+ can only move out through another protein complex called ATP synthase. The diffusion out of the space into the matrix of H+ ions through ATP synthase is called "chemiosmosis." ATP synthase consists of two main components. The part located in the mitochondrial matrix rotates and binds to ADP, Pi, and ATP. As H+ ions diffuse back into the matrix through ATP synthase, they drive the physical rotation of the protein, enabling it to combine ADP with Pi. This phosphorylation of ADP produces ATP in a process known as oxidative phosphorylation.
For each pair of electrons that move from Complex I to Complex IV, about 2.5 ATP can be produced. For each pair of electrons that move from Complex II to Complex IV, about 1.5 ATP can be produced. Therefore, if we round up, it is often stated that each NADH with its electrons and H+ ions yield 3 ATP while each FADH2 will yield 2 ATP. NADH becomes NAD+ at the beginning of the electron transport chain. Also, FADH2 becomes FAD. This recycles these electron carriers such that they can be used again in earlier metabolic reactions. Without NAD+, reactions that use NAD+ cannot occur, the same is true for FAD. ATP and it’s metabolites also play crucial roles, lets visit that briefly.
ATP, ADP, and AMP: Life's Energy Currency System.
As mentioned already, ATP is the “loaded” energy currency that contains three phosphate bonds and holds the most energy. Think of it as a 100-dollar bill. ADP is the “partially spent” form that contains two phosphate groups and can be thought of as a 50-dollar bill. AMP is the “depleted form that contains one phosphate group and can be thought of as the 20-dollar bill. It is the ratio of these molecules that act as the body’s energy gauge. A high ATP:ADP ratio lets the cell know that it is in a good energy abundance and can continue doing energy-intensive tasks like growth, repair, and storage. A low ATP:ADP ratio tells the cell that it is in an energy depletion state which triggers energy conservation pathways and inhibition of some non-essential processes. A high AMP level pulls the fire alarm and triggers rapid energy production and suppresses energy consuming processes. Here are some examples:
During exercise muscles rapidly use ATP so that the conversion of ATP to ADP increases. If the levels are intense, it will result in a rise in AMP levels which will trigger the cells to take in lots of glucose and start drawing on their fat reserves to make more energy. After eating a meal, ATP levels rise and both ADP and AMP levels fall which activates energy storage and growth.