Crossbridge Cycling and Sliding Filament Theory
During muscle contraction, the myosin heads link the thick and thin myofilaments together, forming cross bridges that cause the thick and thin myofilaments to slide over each other, resulting in a shortening of each sarcomere, each skeletal muscle fiber, and the muscle as a whole, much like the two parts of an extension ladder that slide over each other. To summarize, in order for the shortening of the muscle to occur, the myosin heads have three important properties:
In order for these muscle fibers to contract, there needs to be an electrical event (an action potential) that is followed by a mechanical event (the contraction of the muscle fiber). Because you have already learned about the resting membrane potential and the action potential, we'll move past that and talk about the sliding filament model of muscle contraction.
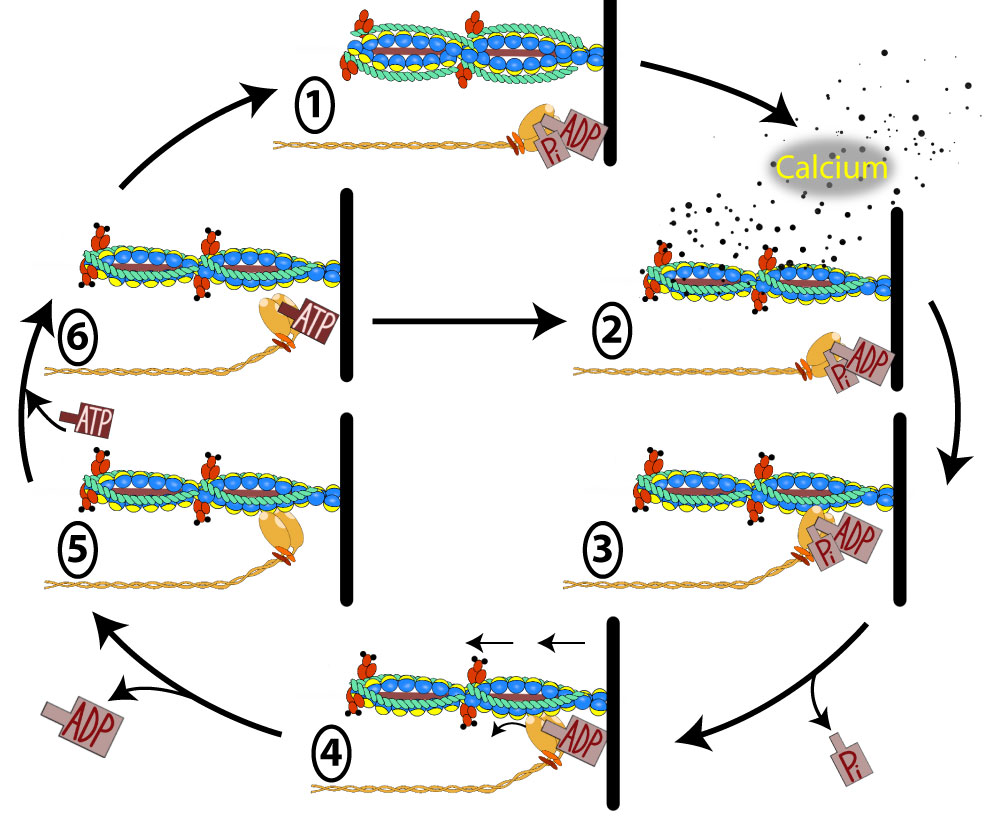
Using the numbers in the image above, we will discuss each step of the cross bridge cycle:
1.
A sarcomere that is in a relaxed muscle will have the myosin head "cocked" back and lying flat. The myosin head will not be attached to the active sites on the actin because the tropomyosin will be covering these active sites which prohibits the myosin from attaching.
2.
Contraction in all muscles is tightly linked to calcium. In fact, if you're into tattoos, you might as well get one that reminds you: every time you say "muscle," the next word out of your mouth should be "calcium." This connection holds true for all muscle types: skeletal, cardiac, and smooth. Calcium is everything to muscles; it not only triggers contraction but also plays a crucial role in muscle growth and fatigue.
Skeletal muscle relies entirely on the sarcoplasmic reticulum for its calcium supply. If an action potential has triggered the DHPR in the T-tubule, we would expect calcium to be rushing out of the sarcoplasmic reticulum (often called the SR) and forming up around the myofilaments of the sarcomere. As the calcium diffuses to the troponin molecules it will attach to them and cause them to go through a conformational change which in turn moves the tropomyosin off of the active sites for the G-actin.
3.
Now that the active site has been exposed on the G-actin, the myosin heads will quickly raise up and bind to the actin.
4.
The binding of the myosin to the actin will cause the myosin head to go through a confirmational change that will result in the inability to continue holding onto the Pi (phosphate) that was bound to the myosin head and it will diffuse away after its bond breaks. The conformational change also involves a moving forward of the myosin head as it pivots on that hinge region. Because the myosin head is bound to the actin, when it moves forward, it will pull on the actin which brings the z-disc closer and shortens the sarcomere. This is called the power stroke.
5.
As the power stroke finishes, the myosin head is in a new conformation that no longer is able to sustain a bond with the ADP that was attached. The ADP is lost and diffuses away. The myosin is now locked in a head forward position and is bound to the actin. At this point, only an ATP could release it (step 6). If no ATP is available, (ie in cell death), then the myosin remains attached until the proteins themselves denature. This is the cause of rigor mortis.
6.
An ATP is likely nearby as a healthy live cell makes ATP continuously. An ATP binds the myosin head which causes the myosin head to let go of actin. At this point, it would be possible for the sarcomere to be lengthened again because there is not attachment of myosin to actin. This explains why we say that ATP is required to relax a muscle. This might seem a little strange that ATP is required to relax a muscle rather than contract it, but ATP is important ultimately for a muscle to contract. Here is how it works. Once ATP causes the myosin to remove itself from the actin, it will be hydrolyzed by the activity of ATPase located within the myosin head. As the ATP is cleaved into ADP and Pi, the energy form this cleavage will be used to cause the myosin head to "cock" back into a high energy straightened position underneath the actin.
If stimulation of the muscle has ceased, there will be no more calcium diffusing around the sarcomere, and the sarcomere will assume the state it was in for step 1.
However, if calcium continues to diffuse around the sarcomere, then we go immediately to step 2 and start the cycle over again.
The steps above explain the confirmations of one myosin through a cross bridge cycle. In reality, there are many myosin proteins all doing this in an asynchronous fashion (They do not all cycle at the same time). This allows a portion of the myosin heads in a sarcomere to always pull on actin and shorten the sarcomere as long as there is enough calcium around. The sarcomere will stop shortening when the stimulus stops releasing calcium from the SR and the SERCA pump is capable of pumping the calcium back into the SR.
Animations can be helpful. Any quick google search using the terms "cross bridge cycle animation" can bring up several good animations. Here is one that is a good example:
https://www.youtube.com/watch?v=Lmxm_9XqDbg