Ions and Cell Membranes
Review of Ions
Electrophysiology, by definition, is the study of the electrical properties of biological cells. It involves measurements of electric current and electrical activity of not only neurons, but virtually all cells of the body. When we talk about the electrical properties of cells, we are not talking about electrons moving through wires like you may suspect. Rather, electrophysiology deals with ions (not electrons) and their movements across the cell membrane. You have learned that atoms consist of equal numbers of positively charged protons and negatively charged electrons, unless they give up or accept electrons, which would make them an ion. When we take an ionic compound like NaCl and place it into water, the two ions dissociate and become free floating Na+ (positive charge, cation) and Cl- (negative charge, anion) ions. These ions (Na+ and Cl-) along with Ca++, H+, and K+ are very important in the human body.
Separating Charges
Opposite charges attract, similar to how Na+ and Cl- ions are attracted to each other to form an ionic compound. If we can somehow separate opposite charges from one another and then keep them separated, but maintain the possibility of reuniting, we can generate quite a bit of energy. Consider what happens when you rub a balloon on your head. When we add work (energy) by rubbing the balloon on the head, we cause a separation of electrical charge. In other words, we cause the electrons from your hair proteins to move from your hair to the balloon surface. The balloon surface gains an attractive force called static electricity. When we put the balloon close to other people's hair, their hair shafts will become attracted to the negative charges on the balloon. Two important things come out of this analogy; first, opposites attract, and second, it takes energy, or work, to separate charges. In the body, the cell membrane has the ability to separate charges (i.e. ions) by performing work. This separation occurs through the action of an ATP driven pump called the sodium/potassium ATPase pump. Every cell of your body expresses this protein for the specific purpose to separate Na+ and K+ ions across the membrane.
Two Chamber Analogy
The figure below represents an artificial system of two chambers (A and B) separated by a semipermeable membrane. Semipermeable means that only certain substances can diffuse across it. We will assume that the membrane is permeable to cations, but not to anions. If we placed two different concentrations of a KCl solution into each chamber, say 0.1 M solution of KCl in chamber A and a 1.0 M solution of KCl in chamber B, what would happen (See figure below)?

Since the membrane is only permeable to cations, K+ will begin to diffuse down its concentration gradient from chamber B (high K+ concentration) to chamber A (low K+ concentration (see figure below).

When will the diffusion of K+ stop? You might be tempted to say, “When the concentration of K+ in chamber A is equal to the concentration of K+ in chamber B.” In the previous section, you would have been correct! But in this section, we are dealing with charged solutes (ions). When a single ion diffuses across a membrane, it creates an electrical gradient. Similar to a concentration or chemical gradient, an electrical gradient is when you have an area of higher charge than another. Look back at the figures above., Initially, no charge differences existed between the two chambers; both sides had the same number of positive and negative ions respectively. However, since the membrane is not permeable to Cl-, and K+ started to diffuse into side A going down its concentration gradient, there is now a difference in charges between the two chambers. Side A now has more positive ions (K+) than negative ions, making it positively charged; and side B now has more negatively charged ions (Cl-) than positive, making it negatively charged (see figure below). With chamber A being positively charged and chamber B being negatively charged, we have now created an electrical gradient. For K+, a cation that is attracted to negatively charged particles (i.e. the Cl- ions), its electrical gradient actually counters its concentration gradient, going from side A to B. This means that it becomes more difficult for K+ to diffuse down its concentration gradient into side A because of the electrical force (positive charge) it generates that opposes more K+ from diffusing across the membrane. Remember opposites attract, so the Na+ is more attracted to the chamber with more Cl- ions.
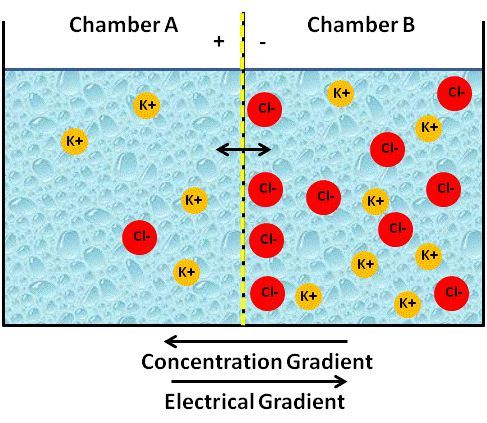
The net flow of K+ will stop when the force of the electrical gradient (i.e. the attraction of the Na+ ions to the Cl- ions) equals the force of the concentration gradient. We call this a state of equilibrium, even though the numbers of K+ on side B will still be higher than side A. When the two forces, the electrical gradient and the chemical gradient, equal each other (equilibrium) and the net movement is zero, we call the state resting and can refer to the two combined gradients as the electrochemical gradient. We have now successfully created a state of charge separation, something we call an electrical potential, or voltage because side B is now negatively charged compared to side A.
This content is provided to you freely by BYU-I Books.
Access it online or download it at https://books.byui.edu/bio_264_anatomy_phy_I/531___ions_and_cell_.

