Adhesive Bonding
When two materials are joined together using adhesive bonding, those two materials are called the adherends. An alternative name for an adherent is a substrate. The material that forms the bond between them is called the adhesive. This section begins with a discussion of adherents that are plastics, for it is the joining of plastic materials for finishing and assembly that is of primary concern. Later, this section examines those adhesive materials that are polymeric and, therefore, are an important application of some polymers.
21.7.1. Processing of Adhesives (the Joining Process)
The first step in the joining process is the preparation of the adherent. The method of this preparation depends strongly on the nature of the adherent and is, therefore, discussed in the following section on adherents.
After the adherents have been prepared, the adhesive is applied. Liquid adhesives are commonly applied by brush, spatula, roller, flow gun, spray, and dip coating. Some of these methods, such as dip coating and spraying, are especially useful for adherents that have complex shapes, whereas rolling and spatulas are better for flat panels. Film adhesives work best on flat panels but can be shaped to fairly complex forms by cutting and applying in strips. Some pressure is required to make sure that the adhesive flows to fill in the inevitable gaps between the strips.
After application of the adhesive, the coated adherents are placed together and held until the adhesive becomes solid. A press can be used to hold the pieces together but jigs and fixtures that keep the alignment of the part and apply moderate pressure are also common. Most adhesives need to be pressurized during cure. If a press is not used, mechanical pressure devices like clamps or springs work well. Another way to exert pressure is with a vacuum bag.
When under pressure or in the jig/fixture, the adhesive solidifies. That step can be done by simply allowing the adhesive to cool (if it is a hot-melt adhesive) or by curing the adhesive (if it is a thermosetting material). During cure the important parameters are temperature and time. These are best determined by consultation with the adhesive manufacturer, but some testing should also be done to ensure that the properties desired of the adhesive are met in the particular application.
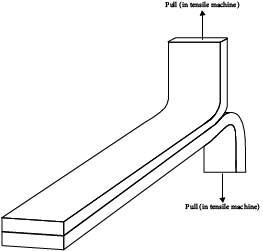
Both destructive and nondestructive testing are common. Because of the particular failure modes of adhesives (as opposed to bulk materials), peel tests are the most important mechanical test. The simplest of the peel tests is the T-peel (named for the shape of the specimen, which is depicted in Figure 21.7). In this test the specimen is prepared by bonding together the substrates (which are shaped appropriately) using the techniques to be employed in the actual process and then pulling the two parts of the specimen apart using a tensile test machine. Other destructive tests include the various lap shear tests and tests that use the exact joint configuration of the actual parts to be joined.
Nondestructive tests include proof-loading (where a specific force is applied that satisfies the requirement of the application but is less than the failure of the part), ultrasonic testing or some other spectroscopic inspection method, and visual and tapping tests to sense the integrity of the bond. Environmental tests are also common. These might include testing after exposure to various environments, including solvents, thermal cycling, UV exposure, and vibrations.
21.7.2. Adherends
Not all plastics can be easily or, perhaps, even successfully bonded with adhesives. The differences between those plastics that are readily bonded and those that are not are based principally on surface characteristics.
A key feature of adherent surfaces that can be bonded easily is that the adhesive covers or coats the surface. This coating of the surface is called wetting. The controlling factors in whether a surface is wetted by a liquid or whether the liquid beads and doesn't wet the surface are the surface energies of the two materials. If the surface energy of the liquid is lower than the surface energy of the solid, then the solid surface will be wetted by that liquid. This rule arises from the free-energy equation introduced earlier in this text. Whenever the net free energy is negative, the phenomenon releasing that energy will occur naturally. Hence, when a solid surface is wetted by a liquid, the free energy is negative or the energy of the system is lowered. This occurs when a highenergy surface is covered so that a lower-energy surface results.
If an adherent surface is not wetted by the desired adhesive, then there will be poor adhesive bonding. This can sometimes be remedied by modifying the adherent surface to increase its energy and therefore allow wetting. The following are some ways to treat or prepare the surface of the adherent and promote adhesive bonding.

Surface Cleaning. Even plastic adherents that have high surface energies and are therefore easy to bond may have surface contaminants that reduce surface energy and interfere with bonding. These surface contaminants can come from the molding operation when mold releases are used to facilitate removal of the plastic part from the mold; from general handling, which imparts grease and oils onto the surface; from plant high-pressure air used to blow debris off the parts when the air has been contaminated with oil; and from general environmental contamination arising from a variety of sources, including the packaging materials and the general dusty conditions within the plant. Whatever the cause of the contamination, it should be removed.
The most common method of removing surface contamination is by wiping or dipping the material in a solvent. The solvent should be chosen to solvate the surface contamination without affecting the plastic adherent and should itself be easy to remove. The solvent may also be required to remove other materials contaminating the surface. Water, for example, interferes with the cure mechanisms of some adhesives and must be removed for good bonding.
Chemical Etching. When the clean surface of the adherent is still not sufficiently energetic to allow wetting by the adhesive, a chemical etch may be useful. In this situation, a chemical that will react with the adherent is applied to the surface. This application changes the nature of the surface and, if the proper type of reaction occurs, the energy of the surface is raised and wetting by the adhesive becomes possible. The chemical etching material is usually removed before the adhesive is applied. If the etched surface is likely to change back or be altered in some way so that the activity is lost, a coating (called a primer) may be applied to preserve the surface characteristics.
Flame Treatment. Many plastic materials, especially the polyolefins, can be treated with a flame to increase their surface energy. Brief treatment with an open flame often causes some oxidation of the surface of the adherent and thereby raises the surface energy. Flame treatment is frequently done on polyethylene and polypropylene to promote bonding and other adhesion-dependent phenomena such as printing.
Corona Treatment. Oxidation of plastic surfaces can also be achieved by passing an electric spark, called a corona, over the surface of the adherent, which then becomes wet table. Corona treatment is generally more aggressive than flame treatment. After corona treatment, the surface may be both oxidized and physically eroded. The polyolefins are the most frequently corona-treated plastics.
Plasma Treatment. Plasma (discussed in detail in Chapter 20) can be used to clean a surface, etch a surface, or react with a surface. It can also be used to cause a reaction (polymerization) to occur in the gaseous phase that would be deposited onto the surface. Plasma obviously has wide capabilities for surface modification of plastics, but the relatively high cost of the equipment and need for operating in a vacuum have limited its use.
Coupling Agents. Some surfaces can best be treated by coating the surface with some material that promotes bonding to the desired adhesive. Such materials are called coupling agents because they couple the surface and the adhesive. Typically, these materials are chemicals that have two different chemical natures, usually one on one end of the molecule and the other on the other end. When one end is chemically like the adherent and the other end is like the adhesive, then the molecule serves to link the adhesive and adherent together. Coupling agents are very common with ceramic adherents (such as fiberglass) but less so with plastic adherents. Nevertheless, using coupling agents remains an interesting and potentially useful method for promoting adhesion.
Mechanical Abrasion. When modifying the energy of the adherent surface is impossible, or when the modifications need enhancement, mechanical abrasion can further increase the ability of an adhesive to bond to an adherent. The surface imperfections (small cracks and pits) created by mechanical abrasion serve as locations where the adhesive can enter and then mechanically intersect with the adherent. No thermodynamic change in the surface is made by this mechanical abrasion. Therefore, wetting is not increased. The bonding relies strictly on mechanical forces.
Some general comments about the chemical nature of surfaces and their energy may be useful in understanding the actions of the various surface treatments just described. In general, surfaces that have pendant oxygen atoms (usually as OH groups or O groups) are very energetic and, therefore, easily wettable and bondable. (The presence of OH and O groups on the surface of an adhesive is also favorable for adhesion promotion in many cases.) When oxidation occurs, such as with the flame or corona treatments, O and OH groups are the chief products created on the surface. Other pendant groups that promote adhesion are groups containing oxygen, such as organic acids, esters, and ketones, NH, CN, Cl, and sulfur-containing groups.
Poor surface adhesion is often associated with having only hydrocarbon atoms on the surface, such as polyethylene and polypropylene, or with surfaces that have tightly held electrons, such as fluorocarbons or ethers. Common greases and oils are chemically similar to the polyolefins and, therefore, adversely affect the bendability of a surface when they are present. Hence, solvent removal is mostly to clean these olefinic (oil-like) materials from the surface.
The actual bonding at the surface can be of three types. Covalent bonds can be created between the adherent and the adhesive, which results in the strongest bonding. The second type of bonding results from an attraction between the adherent and the adhesive, such as hydrogen bonding, van der Waals forces, induced dipole attractions, and so on. These attractions can be quite strong but may also be quite weak, depending on the nature of the materials. The third type of bonding is mechanical bonding, where the adhesive fill in crevices on the surface of the adherent and thereby becomes mechanically entrained on the surface. Mechanical bonding can occur with both of the other types of bonding or it may be the only type of bond that is present.
Ideally, the bond between the adherent and the adhesive will be so strong that if fracture occurs, it will be either within the adhesive itself or within the adherent itself rather than at the junction between them. When failure occurs within a single material rather than at the boundary of the material, the failure is called cohesive. When the failure occurs at the interface or boundary between two different materials, such as between the adherent and the adhesive, the failure is called adhesive. The strongest bonds are those in which only cohesive failure occurs.
21.7.3. Adhesives
When applied to the adherent surface, adhesives are usually liquids but may also be pastes or solid films. In all cases, however, the adhesive goes through a liquid phase and wets the surface of the adherent or is somehow pressed against the surface so that interaction between the adhesive and the adherent occurs. After covering the surface of the adherent, most adhesives go through some chemical or physical change such that they become hard.
(Exceptions to hardening adhesives would be those that are meant to stay flexible, such as rubber cement. In these cases, the only change, if any, is a thickening of the material, normally from a solvent loss.) This hardening step can result from the loss of a solvent, cooling of the adhesive from a melt to a solid, chemical reaction within the adhesive such as crosslinking or polymerization, or pressure against the adhesive that causes some interaction to occur with the adherent.
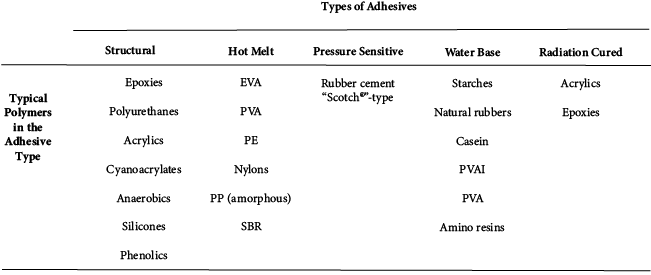
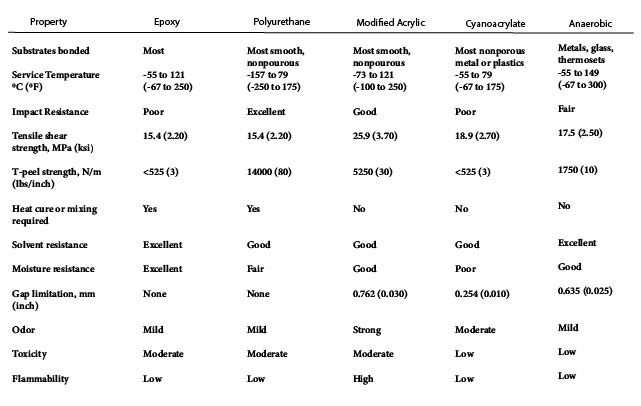
A wide range of adhesives are available to meet the many applications that require adhesive bonding. Adhesives are used to bond plastics, metals, ceramics, composites, wood products, and various combinations of these. The adhesive is chosen for its ability to bond to the adherent and for its other properties, such as structural strength, cost, ease of application, and resistance to environmental effects. For simplicity, adhesives can be divided into five common types (structural, hot melt, pressure sensitive, water base, and radiation cured), each comprised of many different specific materials, but all characterized generally as polymeric. Each of the five general adhesive groups, outlined in Table 21.2, is considered separately. Properties for several selected adhesives are given in Table 21.3.
21.7.4. Structural Adhesives

These are adhesives that can be stressed mechanically and can withstand a variety of difficult environmental conditions such as high temperature, adverse solvents, and creep potential. Structural requirements dictate that most of these adhesives be either one- or two-component thermoset systems. The most important polymeric groups within this class of adhesives are epoxies, polyurethanes, modified acrylics, cyanoacrylates, anaerobics, silicones, phenolics, and various high-temperature polymers.
Epoxies (described in Chapter 9) are the most common of the structural adhesives. Epoxies are generally two-part adhesives, where one part is the epoxy and the other is the hardener (a chemical that will open the epoxy rings on two chains and bond between them). These adhesives can be cured either at room temperature or at elevated temperatures generally up to 350°F (175°C). They are generally brittle and relatively inexpensive.
Table 21.2 Types of Adhesives and Common Polymers within each Type
Polyurethanes are tougher than epoxies, generally maintaining good flexibility even at low temperatures, although high-temperature stability is poor. Polyurethanes are made by combining two reactants, a polyol and an isocyanate, although in some formulations the polyol reactant can be water. Reaction begins immediately after mixing of the components and often occurs without the need for heating. Pot life is, therefore, limited.
Modified acrylics cure by a free radical polymerization method. They differ from standard acrylics in that rubber or other tougheners have been added. Typically, one component is applied to one adherent and the second component to the other adherent. The materials react when the adherents are pressed together. These adhesives can be used on most materials, even when the surface is slightly oily or improperly cleaned. Cure times are quite long. Impact resistance is improved over standard acrylics but is still only good (rather than excellent). Service temperatures are moderate.
Table 21.3 Typical properties of Widely Used Adhesives
Cyanoacrylates (superglues) are single-component systems that cure when in the presence of even small amounts of moisture, as is present in most ambient atmospheres. These Adhesives bond well to most materials, including skin. This can present a safety hazard but is also an advantage when cyanoacrylates are used to suture skin together in surgical procedures. Cyanoacrylates are relatively expensive and strong but quite brittle.
Anaerobics are single-component systems that cure by free radical polymerization. The polymerization reaction is inhibited by oxygen, so no reaction will take place until oxygen is removed from the system. The air is excluded from the system when the coated adherents are pressed together. Therefore, by mating the parts the reaction is initiated and proceeds rapidly. Toughness and strength are moderate.
Silicones are available as either one- or two-component systems. The one-component systems cure on contact with atmospheric moisture. Silicones can be formulated to cure at room temperature (RTV) or at high temperature (HTV). They have good adhesion over a wide temperature range, - 76° to 480°F (- 60°C to 250°C), and can go as high as 700°F (370°C) for some formulations. Silicones are tough and flexible with good solvent, moisture, and weathering resistance.
Phenolics and urea formaldehydes are low-cost thermosetting systems available in one- or two-component systems. They are generally strong but brittle. Phenolics are dark colored, which limits applications. These Materials dominate the plywood and chipboard markets.
Hot-Melt Adhesives. Hot-melt adhesives are thermoplastic materials that are heated to a melt, applied to the adherents, and then allowed to harden by cooling. The most important thermoplastics used for hot-melt adhesives include ethylene vinyl acetate (EVA), polyvinyl acetates(PVA), polyethylene (PE), nylons, amorphous polypropylene, and various block copolymers, such as those between styrene and butadiene. Melt viscosity is an important factor in the application of these materials. Therefore, a key to their successful use is a proper heating/dispenser unit. For industrial applications, the hot-air welding tool (shown in Figure 21.8) is very common. In this device a rod of the solid hot-melt resin is fed into a shaping head where it is melted by hot air that is passing through the machine. The molten material is applied to the adherent as the shaping head passes over the surface.
Automatic hot-melt dispensers are used in commercial applications where high speed and repeatable application are required. These automatic dispensers have a chamber in which the plastic resin is placed and kept hot. The dispensers have a pumping system that moves the material from the reservoir in the heated chamber to the application nozzle. Then, when the signal is given (usually from some timing device), the pump moves the hot melt onto the surface in the quantity desired. Physical and mechanical properties for hot melt adhesives are the same as in their plastic form; these are given in Appendix 3.


21.7.5. Pressure-Sensitive Adhesives
These materials, which are often supported on a tape or some other backing material, are tacky at room temperature and adhere when brought into contact with the adherent through some pressure. These materials must be highly viscoelastic so that they flow when pressed together, but they also must resist excess flowing, have some elastic nature, store bond-rupture energy to provide peel and tack, and dissipate energy during adhesion. The highly elastomeric nature of many of these materials has earned some of them the name rubber cement. The viscoelastic nature of the adhesives is clearly demonstrated when trying to remove a label that is bonded with a pressure-sensitive adhesive. If the label is pulled quickly, the adhesive resists the movement and the label breaks. If the label is pulled slowly, the adhesive gradually separates and the entire label can be removed, hence demonstrating the time dependent nature of the adhesive.
21.7.6. Water-Based Adhesives
Many of these materials are natural products that are combined or occur naturally in combination with water (latex materials). Some typical examples are starchbased adhesives, latex rubber, casein (from milk products), animal glues, sodium carboxymethylcellulose, and sodium silicate. Non Natural materials that are also water-based adhesives include polyvinyl alcohol, polyvinyl acetate, amino resins, block copolymer, sand several rubbers. The waterbase adhesives have lower strength than most of the other types of adhesives but are nontoxic, are nonpressure sensitive, and can occasionally be dissolved in solvents other than water. When used, the solvent must be removed to affect the bonding.
21.7.7. Radiation-Cured Adhesives
These are generally free radical cure materials that are placed between the adherents and then radiated to cure. The radiation source is usually either a UV light or an electron beam. One of the substrates must be transparent to the radiation. These materials can be as strong as structural adhesives. The most common applications are in electronic equipment magnetic tapes, floppy disks, and dental and medical devices.