Copolymers
Some unique polymers can be created by mixing together more monomer types than the minimum required to effect normal polymerization, such as mixing ethylene monomer and propylene monomer. Normal addition polymerization requires only one type of monomer to create a polymer chain, whereas normal condensation polymerization normally requires two monomers, each with two active ends. When more than these minimum numbers of monomer types are mixed, the polymer will contain some combination of the monomer types present. Polymers with mixed monomers are called copolymers, and they are, therefore, characterized as having more than one type of repeat unit. (When only the minimums of monomer types are present, that is, when the normal polymer is made, the polymer is called a homopolymer.)
Copolymers in four general patterns can be made by both the addition and the condensation polymerization methods. These are illustrated in Figure 2.27 for the simpler case of addition polymerization. Some commercial copolymers, all to be discussed later in this text, are acrylonitrile-butadiene-styrene (ABS), styrene-acrylonitrile (SAN), and ethylenevinyl acetate (EVA). (Some copolymers can be referred to as terpolymers, which reflects that three monomer types are polymerized together.) The determination of which type of copolymer would be formed is dependent upon the basic nature of the monomers and their mutual reactivity, the conditions of the reaction (such as concentrations, temperatures, catalysts, etc.), and any specific modifications that might be made to the monomers or polymers allowing the copolymerization step to be activated as desired. All these conditions are used for various copolymerizations.
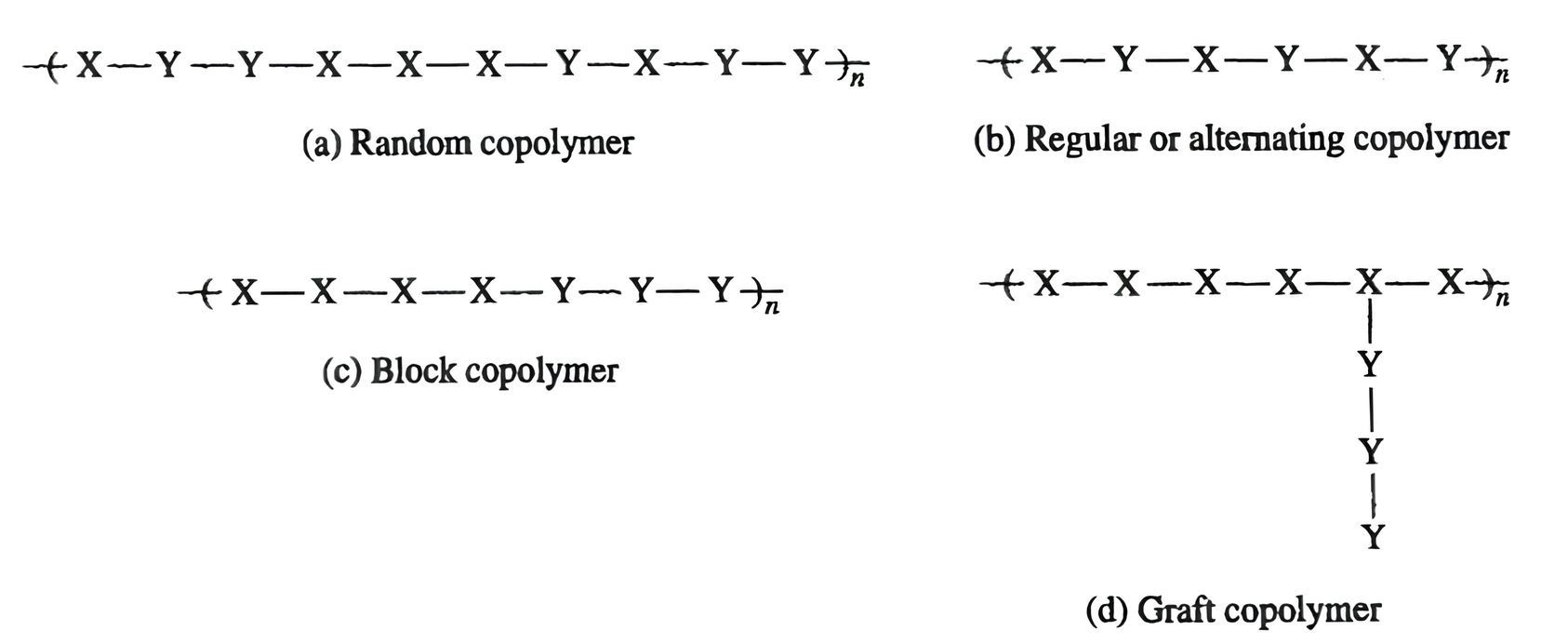
Figure 2.27 Copolymer patterns for addition polymerization.
One monomer, X, which could polymerize alone, is mixed with another monomer, Y, which could also polymerize alone. The mixture of monomers can produce a random copolymer structure (Figure 2.27a), where there is no pattern in the order of the monomers along the polymer chain. This is the most common copolymer pattern.
Another pattern could be a regular or alternating copolymer pattern, as shown in Figure 2.27b. In this pattern the monomers have a regular, alternating sequence, which can be represented as X-Y-X-Y, etc. This polymer should not be confused with a normal condensation polymer, although the alternating pattern is the same. In the copolymer case illustrated, two addition monomers were present (one more than the number needed to polymerize), so a copolymer was made. Because copolymerization requires more than the minimum number of monomers, a copolymer in a condensation polymerization requires three monomers, and the resulting polymer would require at least the pattern A-B-C, whereas the regular polymer pattern is just A-B.
A third type of copolymer pattern occurs when long sequences of one monomer join long sequences of the other monomer to form the chain. This pattern is called a block copolymer, shown in Figure 2.27c. This pattern is characterized by several monomers of one type in a row along the backbone followed by several monomers of the second type, also along the backbone, such as X-X-X-Y-Y-Y-X-X, etc.
The fourth pattern is a graft copolymer (Figure 2.27d). In this type of copolymer, a polymer chain is formed by one monomer (X) and then a chain of the other monomer (Y) is attached as a branch to the main backbone.
In general, the properties of random and alternating copolymers are averages of the properties of the two homopolymers. The properties of block and graft copolymers are not averages but have some characteristics like one homopolymer component and some properties like the other homo-polymer.