Electrical Properties
The basic electrical properties of all materials are related to the ease of formation of a conductive path through or along the surface of the material. In some materials, such as metals, the sea of electrons that bind the metal ions together can move freely throughout the metal structure and thus provide a conductive path. Metals are, therefore, highly conductive. In other materials, charged atoms (ions) can move freely within a solution, such as salt dissolved in water. These, too, are conductors.
The electrons and ions in plastics and ceramics are much more restricted except in some special cases of conductive polymers, which are discussed later in this section. Therefore, plastics are not usually conductive to electricity but are, instead, insulators. Some caution should be exercised, however, in using the terms conductor and insulator to describe any particular material because the terms are really comparative in nature and not absolute. For instance, most plastics would normally be considered as insulators but that is only as compared to common conductors such as metals. A specific plastic material could be considered a limited conductor if compared to some other plastic material with much lower conductivity. PVC is, for instance, an insulator when compared to copper but is a conductor, or at least is more conductive, when compared to PTFE.
Several electrical properties measure the tendency of a material to form a conductive path under different conditions. The most common of these properties are resistivity, dielectric strength, arc resistance, dielectric constant, and dissipation factor.
5.6.1. Resistivity
The resistivity of a material presents to the flow of electrical charge. This is sometimes called ohmic resistance because it can be expressed or quantified by the simple Ohm's law, that is, the voltage (V) is equal to the current (I) times the resistance (R), as shown in Equation (5.6).
The resistivity can be measured through the thickness of the sample and is then called the volume resistivity, or it can be measured along the surface of the sample and is then called the surface resistivity. Plastics have much higher volume resistivities than do metals, as illustrated in Table 5.3.
| Material | Volume Resistivity Range (Ω · cm) |
| Fluorocarbons (PTFE) | 1018 |
| Polystyrene | 1017 |
| PVC | 1015 |
| PVC (plasticized) | 1011 |
| Phenolics | 1011 |
| Antistatic Polymers | 107-1013 |
| Static-dissipator polymers | 103-106 |
| Conductive polymers | 10-5-103 |
| Metals | 10-5-10-6 |
Table 5.3 Volume Resistivity for Various Materials.
The resistivities measured by standard (ASTM) tests may be considerably different from resistivities under actual use. The standard test values are measured using controlled specimen size and shape, surface cleanliness, and moisture content. In actual use the parts are almost always of a different shape; will often have surface contaminants such as mold release, solvents, or oils; and may have absorbed considerable moisture, especially if the polymer is moisture-sensitive, such as nylon or cellulosics. The higher conductivity (lower resistivity) of the plasticized PVC compared to unplasticized PVC illustrates that the presence of non-plastic plasticizers, fillers, pigments, or other additives can also make a significant difference in the electrical conductivity of the base plastic material. The values given in Table 5.3 are, therefore, only guidelines for relative resistivities. The test for volume and surface resistivity is ASTM D 257. In this test a low voltage is applied across the thickness of along the surface of a sample under standard conditions.
The primary function of most plastic materials in electrical applications is that of an insulator. This insulator, or dielectric, separates two field-carrying conductors. Typical electrical applications of plastic materials include plastic-coated wires, terminals, connectors, industrial and household plugs, switches, and printed circuit boards. The major requirements of an insulator are to (I) have a high enough dielectric strength to withstand an electrical field between the conductors, (2) possess good arc resistance to prevent damage in case of arcing, (3) have high insulation resistance to prevent leakage of current across the conductors, (4) maintain integrity under a wide variety of environmental conditions, and (5) be mechanically strong enough to resist vibrations, shocks, and other mechanical forces. The key electrical properties embodied in these requirements are dielectric strength, dielectric constant, dissipation factor, volume and surface resistivity, and arc resistance, which are all discussed later in this chapter.
The reciprocal of the resistivity is the conductivity. Therefore, metals are seen to have many times higher conductivities than most plastics. Conductive polymers are a new class of recently developed materials now being used in applications in place of some traditional metal conductors. The development of conductive plastics is an interesting story, which is given in Case Study 5.2 at the end of this chapter. Conductive polymers are made by using monomers that, when polymerized, allow the electrons to move relatively freely along the polymer chain. These electrons are said to be delocalized. One method of achieving delocalized electrons is to have a series of carbons with double bonds between every other pair of carbon atoms, as illustrated in Figure 5.4a. This arrangement of alternating double bonds is called conjugated bonds. The electrons in the conjugated bonds are relatively free to move along the polymer chain and therefore make the polymer much more conductive than other traditional plastics without conjugation. However, if a material is added that creates molecular "holes" in the polymer structure, the ability of the conjugated electrons to move is further enhanced and conductivity goes up tremendously. The process of adding these "holes" is called doping and is done by carefully diffusing a special type of atom into the polymer structure. In the case of polyacetylene, the new atom was iodine, which because it lacks one electron from being a complete octet creates a place (hole) into which the conjugated electrons can flow. The overall effect of using conjugation and doping is that conductive polymers have been made that are about as conductive as copper.
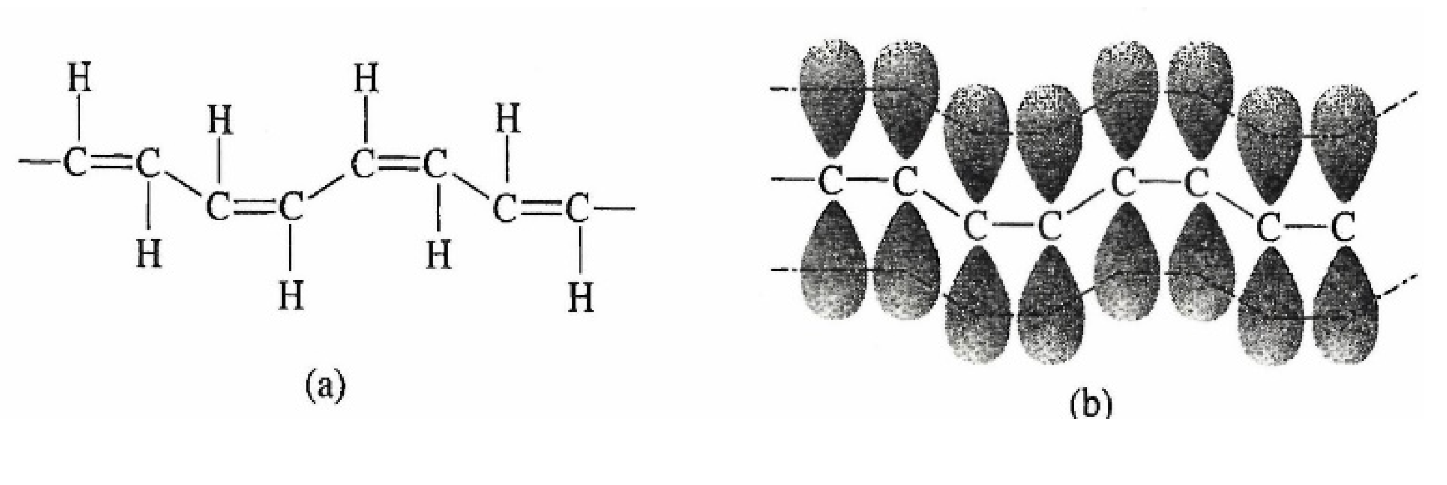
Figure 5.4 Polyacetylene, a conductive polymer showing bond view (a) and atomic orbital view (b) (some Hs omitted for clarity).
Conjugated double bonds are also found in aromatic molecules such as benzene, as described in Chapter 2. The conjugation in benzene is limited to only the ring atoms and therefore the electrons are delocalized only over the ring itself. However, some additional delocalization can be achieved by linking linear conjugated double bonds onto the benzene molecule. Several conductive polymers using benzene-like molecular arrangements have proven to give very highly delocalized electrons and are the basis for new conductive polymers that are, in many ways, improvements over polyacetylene, just as other aromatic polymers have some improved properties over aliphatic polymers. The number of new conductive polymers continues to grow and with that growth, several new applications are emerging.
Some applications for the new conductive polymers have combined the flexibility, light weight, and low cost of plastics with conductivity expected previously only from metals. The ever-increasing need for portability in electronics requires improved batteries. The tremendous growth in the use of laptop computers, cellular phones, and personal digital assistants makes replacing heavier metal components with lightweight polymers highly desirable. Generally, lower cost and occasionally better performance have also been achieved using plastic conductors in batteries. Another major use for conductive plastics is silicon replacement. It is hoped that the performance of silicon devices (such as transistors) can be duplicated or exceeded by conductive polymers at a much reduced price. The ultimate goal is to make integrated circuits using conductive polymers. The current cost of silicon in the purity needed for these applications is about $500 per pound, whereas the cost of an equivalent conductive polymer would be about $10 per pound. Conductive polymers have also been made into devices that provide an alternative to conventional backlit liquid crystal displays (LCD). These displays use conductive polymers in sandwich-type structures in which the active polymeric film layer lies between a semitransparent anode and a back-row cathode. The layers emit uniformly and are tough and flexible, thus greatly enhancing the ruggedness of the LCD. Such devices have already found application in cellular telephones, personal digital assistants, and displays for home appliances.
One interesting property of many conductive polymers is that they swell when they conduct. This means that conductive polymers can change electrical signals into mechanical energy, similar to piezoelectric materials. However, in contrast to piezoelectric films, conductive polymeric films work well at low voltages (about 1/10 as much voltage), thus expanding the applicability of such devices.
Solder is used heavily in the electronics industry to physically and electrically attach electronic devices to printed wiring boards. The solder used is a mixture of tin and lead, which is a concern because of the amount of lead in landfills. Conductive polymers that are adhesives are now being investigated as replacements for solder. Today's printed circuit boards are actually etched metal layers, not printed. However, with conductive polymers, the possibility exists to make truly printed circuit boards, which would dramatically lower the costs associated with making these boards.
Another molecular arrangement that has significant delocalization is graphite. Graphite is a structure in which the carbons are bonded in flat sheets that are then stacked upon each other. The flat sheets have delocalization across the sheet, but not from one layer to the next. Graphite can also be made into fibers (called carbon or graphite fibers), which results in essentially linear conductivity. However, the conductivity of graphite plates or fibers does not approach the conductivity of the delocalized and doped conductive polymers like polyacetylene.
Another method of making a plastic material conductive is to simply add a conductive filler. Many materials will work, including most metal powders, many plasticizers, and many inorganic fillers. The properties of the plastic material when these fillers are added would be consistent with the properties expected when other traditional fillers are added.
5.6.2. Dielectric Strength
As indicated previously, the resistivity of a material is determined under a relatively low voltage. If the voltage is steadily increased, a point will be reached when the electrical force on the electrons within the material is so great that there is an electrical breakdown and a conductive path is formed. The voltage at which this occurs is called the break-down voltage, which, when divided by the thickness of the sample, is called the dielectric strength. The dielectric strength is an important measure of the stability of a plastic insulator in various electrical environments, especially high-voltage applications where plastic insulators are used to separate high-voltage wires. (ceramic insulators are also used for this application.)
The most common test for measuring the dielectric strength of a material is ASTM D 149. In this test a voltage is applied across the thickness of a sample, which has been carefully conditioned to a standard temperature and moisture content. The voltage is then increased until the sample is forced to conduct, usually by the internal heating caused by the high electrical field imposed. Because the thickness is an important factor in determining the voltage required to cause conduction, the results of the test are normalized to a unit thickness by reporting the dielectric strength in volts/mil.
Typical dielectric strengths of plastic materials would be in the range of 20 to 50 kV/mm, which is approximately 2 to 10 times higher than ceramic materials and, of course, hundreds of times higher than metals, which are not insulators, but conductors.
5.6.3. Arc Resistance
The arc resistance is the property that measures the ease of formation of a conductive path along the surface of a material (rather than through the thickness of the material as is done with dielectric strength). In ASTM D 495, a constant, high voltage is applied to the surface of a clean, dry sample and the time to form a conductive path is measured. This property is important in switches and various electronic housings where the rapid opening and closing of electrical contacts can cause sparks that result in surface erosion or breakdown. Another important application requiring good arc resistance is for stand-off insulators that keep the high-voltage wires insulated from the towers that suspend them.
Aromatic materials (those containing the benzene ring) have a tendency to char and thus form graphite like structures that are far more conductive than the original material. These types of materials would have arc resistances of 10 to 150 seconds. On the other hand, nonaromatic (aliphatic) materials have arc resistances of 150 to 200 seconds. PTFE has the highest arc resistance, at over 200 seconds. Some fillers, such as aluminum trihydrate, have been shown to increase the arc resistance of plastic materials.
5.6.4. Dielectric Constant
Dielectric constant or permittivity is a measure of how well the insulative material will act as a dielectric in a capacitor. This constant is defined as the capacitance of the material in question compared (by ratio) with the capacitance of a vacuum. A high dielectric constant indicates that the material is highly insulative, thus permitting the use of small (thin) dielectric material in capacitors. Dielectric constants are dependent upon temperature and the frequency of the alternating electric field that is applied to the sample.
The test for dielectric constant or permittivity is ASTM D 150. In the test, a carefully conditioned sample is placed between plates that are then charged, as they would be in a capacitor. The leakage current is measured and compared to the situation that would exist were a vacuum to exist between the plates.
Nonpolar polymers would generally have a dielectric constant in the 2-3 range; polar polymers can range up to 7. (Pure water, by comparison, has a dielectric constant of 80.) Some applications require a high dielectric constant (such as a microwave dish), whereas others require a low dielectric constant (such as a capacitor). In either case, the ability of the plastic material to dissipate the charge placed upon it is also important. This property is called the dissipation factor.
5.6.5. Dissipation Factor
The dissipation factor of a material measures the tendency of the material to dissipate internally generated thermal energy (heat) resulting from an applied alternating electric field. This heating is caused by the movement of the electrons and atoms within the material in response to the changing polarity of an alternating electrical field. Polymers with highly polar groups tend to have high dissipation factors because they move more in an imposed electric field (assuming all other factors, such as size, are constant). Amorphous structures would be more easily moved than crystalline structures and would be expected to have higher dissipation factors. Another factor affecting the dissipation is the temperature of the material. The dissipation factor for plastic materials increases dramatically above their glass transition temperature because the ability of the materials to move is greatly increased. The dissipation factor is related to the dielectric constant because of the tendency of materials to discharge a capacitor when they heat. Hence, the charge is dissipated easily if the dissipation factor is high. The dissipation factor test is ASTM D 150.
5.6.6. Summary of Electrical Properties
All the electrical properties of plastic materials are used to determine the behavior of parts under various electrical environments and applications. Plastic materials are, in general, highly insulative (non-conductive) when compared to metals. Plastics are therefore convenient materials for capacitors and for other dielectric applications. Hence, plastics are used extensively as wire insulation, circuit boards, capacitor dielectrics, and EM! shielding when other plastic properties, such as formability or low radar detectability, are desired. Ceramics are also insulators and can be used in many of these applications. Ceramics are, however, brittle materials and so applications that require flexibility, such as wire insulation, would not be appropriate for ceramics.
Some new plastics have conductivities high enough that they are used as conductors in special applications where metals are not desired. Some of the new plastics may even be used as semiconductors. Semiconductors must be "almost" conductors; that is, the electrons in semiconductors must be able to be excited with little energy into conductive pathways.
Ohmic conductance in plastics can be achieved by delocalization of the electrons. This is usually done by conjugating double bonds and by the addition of dopant materials. Capacitive conductance is achieved by adding polar groups to the polymer and by increasing the ability of the polymer chain segments to move, such as in an amorphous, open structure, or by increasing the temperature of the material.