High-Performance Thermoplastics
High-performance thermoplastics are distinguished from the engineering thermoplastics because of one or more exceptional properties, usually at a premium price. These polymers are used because of their high performance in the specific applications in which a plastic may be the only viable choice. Most of the high-performance thermo-plastics have been used as the base resin for composite materials in which long fibers (usually carbon or fiberglass) are mixed with the melted resin. These advanced thermoplastic composites compete with thermoset composites for applications in aerospace, high-performance sporting goods, and medical devices.
Every polymer in the class of high-performance thermoplastics has a polymer backbone structure that is chiefly composed of benzene rings or substituted benzene rings that are linked by various functional groups or atoms. This highly aromatic nature provides very high strength, high temperature performance, usually high impact toughness, low flammability (because the polymers tend to char), and reasonably good solvent resistance. Some applications for high-performance thermoplastics are pictured in Photo 8.9.

Photo 8.9 Various high-performance thermoplastic products. (a) PSU flow meter. (b) Polyimide part. (Courtesy of BYU)
The high-performance polymers can be subdivided into families on the basis of the type of bonding between the aromatic rings. Each group will be identified and then the variations within the group for specific polymers explained.
8.9.1. Polyphenylenes (PPE and PPS)
The phenylene bonding pattern is two benzene rings or benzenes with substituent groups joined by a divalent (2-bonding) atom, such as oxygen or sulphur. These materials are thermally stable, solvent resistant, and flame retardant, with somewhat high molding temperatures but low shrinkage and warpage. The moldability has been further improved in polyphenylene ether (PPE) by blending with high-impact polystyrene. The impact strength of these polymers causes them to be more widely used than engineering thermoplastics in applications such as pump impellers and housings, radomes, small appliance housings, video display terminal housings, electrical sockets, valve components, flow meters, wheel covers, and window frames.
PPE has a lower water absorption than any of the common engineering thermoplastics except the fluoropolymers. In spite of the good solvent resistance, these materials can be solvent welded, especially at elevated temperatures, by using chlorinated solvents. The polyphenylenes can also be metal coated, which has led to some automotive applications where thermal stability, dimensional stability, and fatigue resistance are required. Brand names of these materials include Noryl® (GE) and Ryton® (CPChem).
8.9.2. Polyaryletherketones (PEEK, PEK, and Others)
The polyaryletherketones have benzene rings joined by either oxygen in ether linkages or by carbon and oxygen in ketone linkages. The properties are, therefore, similar to those of the polyphenylenes linked by oxygens or sulphurs. These linkages are illustrated in Figure 8.10. The name of polymer simply describes the linkages along the backbone. For instance, if only one ether linkage and one ketone linkage are present, as is the case in the polymer shown in Figure 8.10, the polymer would be called polyetherketone (PEK).
If two ether linkages and one ketone linkage are present, the polymer would be called polyetheretherketone (PEEK). PEEK is the most common and widely sold member of the polyaryletherketone group. The ether linkage favor processibility and the ketone group gives stiffness to the backbone, resulting in strength and high modulus. Therefore, by adding the ketone group to what would otherwise have linkages like the polyphenylenes, the molecule becomes somewhat stiffer and stronger than the polyphenylenes and has higher use and melting temperatures. PEEK has excellent impact strength and chemical resistance and is used extensively as a resin in carbon-fiber-reinforced composites for the aerospace industry.
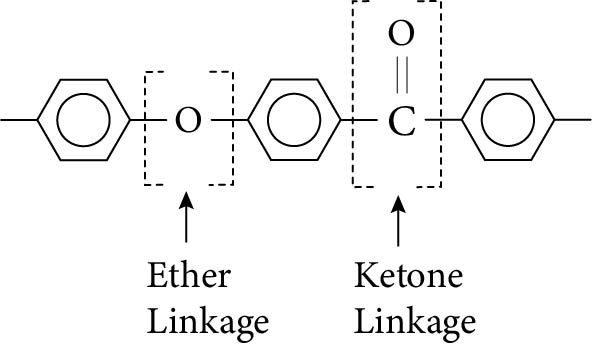
Figure 8.10 Ether and ketone linkages between aromatic groups that are characteristic of the polyaryletherketones.
8.9.3. Polysulfones (PSU and PES)
Polysulfones have aromatic groups, usually with more than one benzene ring, joined by an SO2 group. In some cases, the multiple aromatic rings are directly joined (PSU) and in others they are joined by an oxygen (PES). The SO2 group adds stiffness and strength to the molecule, much like the ketone group does in PEEK. The polysulfones therefore have high stiffness, high temperature stability, and high strength.
The polysulfones are more dimensionally stable and tougher than the polyphenylenes, and about the same as the polyaryletherketones. Creep is very low in these polymers over a wide range of temperatures allowing them to be used for hot water pipes, instrument parts requiring toughness and chemical resistance, circuit breakers, circuit boards, automobile applications near the engine, and interior dishwasher components. The polysulfones compete in many applications against high-performance thermosets, but because the polysulfones can be injection molded, the total part cost can be less because of the reduced time to fabricate by injection molding versus the cure method required with thermosets. The costs of these resins are higher than the polyphenylenes but somewhat lower than the total cost of a metal when the ease of fabrication and the possibility of combining many metal fabrication steps into one molding step are considered.
The polysulfones are noncrystalline and can be clear in some formulations, thus increasing their applicability in appliance housings and high-temperature view ports. Some caution should be used, however, since these materials will solvent craze, and physical and optical properties could be reduced.
8.9.4. Thermoplastic Polyimides (PI and PAI)
The polyimide group adds considerable stiffness to the backbone because it is composed of a benzene ring to which a ring of carbons, nitrogen, and oxygens is attached, as shown in Figure 8.11. This imide group restricts the movement of the backbone and increases the amount of energy required to cause melting. Consequently, polyimides have very high melting points, high use temperatures, and high dimensional stability. They are also very difficult to process because their high melting points are very near the decomposition temperatures. In some polyimides, the imide groups are attached to the rest of the chain with amide bonds. These are the polyamideimides (PAI).
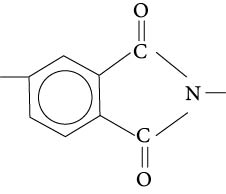
Figure 8.11 lmide group that is part of the polyimides (Pl) and the polyamideimides (PAI).
Brand names for PAI include Torian® (Amoco) and for Pl, Vespel® (DuPont), Kapton® (DuPont), and Kinel® (Rhone-Poulanc). Torian is a molding resin that is processible by conventional thermoplastic methods. Vespel is a molding resin that can only be processed by sintering and other solid-phase consolidation methods. Both of these materials are used for high-performance gears, sliding parts such as bearings, and coatings because the coefficient of friction is quite low for the polyimides. In some cases, these materials, although not as good, compete with the fluoropolymers for nonstick applications, but the adhesion to other materials and the toughness and scratch resistance are much better. Fluoropolymers or molybdenum disulfide can be added to PAI and Pl to make them self-lubricating. Kapton and Kinel are usually sold as films to be used in electrical and high-temperature applications.
Processing of all these materials is difficult. They have very high viscosities and they take a long time to flow. There is some evidence of skin irritation with members of this group, so precautions against long-term exposure to the liquids should be taken.