Polyamides or Nylons (PA)
The polyamides were the first engineering thermoplastics produced specifically by design as a plastic and are the largest family in both production volume and number of applications. Originally the name nylon was associated with and coined by the DuPont company to represent the family. It has since become a synonym for all the polyamides, without regard to the producing company. The major producers of nylon resin have their own brand names for their polymers, some of which are Zytel™ (DuPont), Ultramid™ (BASF), Torayca™ (Toray), Durethan™ (Bayer), Capron™ (Allied Signal), and Akulon™ (Akzo). The differences in engineering thermoplastic properties are more pronounced from company to company than are the differences between commodity thermoplastics, especially because of the wide range of specific resin types.
8.3.1. General Polyamide Family Characteristics
The polymer repeating unit that represents the family of polyamides is shown in Figure 8.1. The subscripts a and b in the repeating unit indicate that in the nylon family the number of CH2 groups can vary from one particular nylon type to another, and it is these variations that are the principal difference between the different types of polymers within the family. These differences are explored in detail later in this section, after the general family characteristics are discussed.
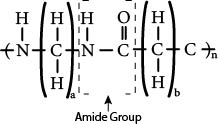
Figure 8.1 Polymer repeating unit for polyamides, where the subscripts a and b can vary depending upon the type of monomers used and n represents the number of repeating units in the polymer chain.




Photo 8.2 Various products made from nylon (polyamide). (a) Tent. (b) Zippers. (c) Gears. (d) Carpet samples.
Nylons are formed by condensation polymerization from reactants (monomers) that combine to make amide groups with water as a by-product. The reactants that form amide groups are the acid and amine functional groups identified in Chapter 2. The amide group, which is formed in the polymerization reaction and is present in each of the polymer repeating units, is the major determiner of nylon family properties. Both the N—H bond and the C—O bonds are polar, with the N and the O being the negative ends. This polarity induces the formation of secondary bonds between adjacent nylon molecules. These secondary bonds (hydrogen bonds) restrict the movement of the nylon molecules relative to each other, thus increasing the tensile strength. The secondary bonding also facilitates the close packing of nylon molecules, resulting in high crystallinity. This crystallinity leads to high strength, high stiffness, good toughness, low gas and vapor permeability, translucency rather than transparency, a sharp melting point, good abrasion resistance, good fatigue life, and high-temperature processing. Typical nylon products that utilize these properties are fibers (for hosiery and durable fabric like tents, ropes, parachutes, and carpets), gears, rollers, shafts, impellers, bearings, films for vacuum systems, cooking bags, and zip fasteners (zippers). Some of these applications are shown in Photo 8.2.
The polarity of the amide group makes nylon sensitive to polar solvents such as water. Water absorption of nylon (approximately 2.5% by weight) is higher than that of most of the other engineering thermoplastics and can have a significant effect on the properties of nylon. For instance, the tensile strength and tensile modulus can decrease by 20% with water absorption. The tendency for nylon to absorb water increases significantly with temperature, so applications where the nylon would be in hot water should be avoided. On the other hand, in many applications in which nylon is exposed to only cold water for brief periods, its properties are not seriously affected by the water absorptivity. For instance, nylon is widely used for ropes, which can often get wet, and the properties are not seriously affected. Absorptivity of water is a problem in processing because nylon resins must be dried before undergoing injection molding. This drying requirement is not, however, unusual for the engineering thermoplastics.
The polarity of the amide group increases the ability of nylon to be joined with traditional solvent-based adhesives. Many effective adhesives for nylon are commercially available. Nylon can also be solvent welded by simply subjecting the surfaces of the two parts to a solvent for nylon. The solvent will soften the surfaces, and while still soft, the parts can be joined and clamped and then dried. Just as the polarity of nylon increases sensitivity to polar solvents, it decreases sensitivity to nonpolar solvents. Therefore, nylon is used extensively in applications in which the plastic part is subjected to oil and grease.
Other materials are usually the preferred choice in electrical applications because the resistivity of nylon is strongly affected by the moisture content.
The sharp melting point of nylon with the accompanying low viscosity of the melt are advantages in injection molding but disadvantages in extrusion and blow molding, where melt strength is required. When nylon is extruded, wide molecular weight distributions should be used and the temperature profile of the extruder should be lowered at the exit so that the viscosity of the melt can be maximized.
8.3.2. Properties of Specific Nylon Types
The differences among the various types of nylons largely depend on the number of carbons in the molecular segments between the amide groups. These segments are simply the CH2, units existing in the monomers used to form the nylon polymer. Hence, if the nylon is made from a diamine made up of six carbons, as shown in Figure 8.2a, and a 12-carbon diacid, as shown in Figure 8.2b, the resulting polyamide would be named nylon (6/12), which is pronounced "nylon-six-twelve." (The number of carbons in the diamine is given first followed by the number of acid carbons in the carbon number designations.) Polyamides can also be formed from just one monomer if that monomer has an amine group on one end and an acid group on the other. Such a monomer is shown in Figure 8.2c. The resulting polyamide from the combination acidamine would have six carbons between each of the amide groups but, because only one monomer is used, the polymer name would be nylon (6). Nylons of many different types are being sold commercially, including nylon (6), nylon (11), nylon (12), nylon (6/6), and nylon (6/12). The most widely used of the different types is nylon (6/6), which was also the first made.
The choice of six carbons in both the diamine (hexamethylene diamine) and the diacid (adipic acid) to make nylon (6/6) was probably dictated by the low cost and availability of the monomers. This choice has proven to be a good one for achieving a compromise of properties. The six-carbon segments provide enough flexibility to give nylon (6/6) good toughness and yet allow the polymers to still be stiff, strong, and crystalline. Longer segments are much more flexible. Shorter segments are stronger and stiffer but more brittle. Applications for nylon (6/6) include bristles, sheets, rods, tubes, coatings, molded industrial parts, and many others.
Nylon (6/6) has proven to be nearly ideal for making nylon fibers, which are used for such applications as hosiery, carpets, reinforcement for hoses, belts, and tires, and for other heavy-duty industrial applications. Industrial and carpet fibers must be stiff, strong, tough, and resistant to cracking under severe bending. Other fibers may be softer, more easily colored, or less expensive, but for carpets and industrial fiber purposes, nylon (6/6) fibers are usually superior. One drawback to nylon (6/6) fibers is their strong tendency to build up static charge, which can be reduced by including metallic fibers in the carpet to conduct the electricity to ground and treating the surface with an anti-static agent to prevent or decrease the buildup of the charge.
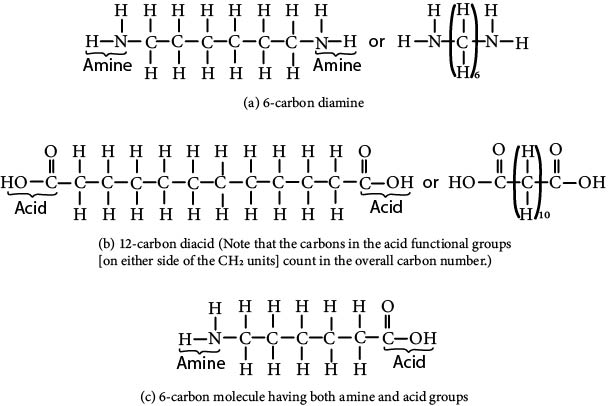
Figure 8.2 Monomers that can be used to make polyamides (nylons). Monomer (a) can react with monomer (b), whereas monomer (c) can polymerize by itself.
Water absorption is affected strongly by the length of the CH2 segments in the nylon molecules. When the number of CH2 units in the monomers is small, the number of amide groups per unit of length is high and so the overall polarity in the plastic is relatively higher. This results in more water absorption. Therefore, shortening the CH2 content, such as in nylon (2/2), will add strength and stiffness but will also increase the amount of moisture sensitivity. Conversely, if a nylon is desired that will make a softer, more flexible fabric (perhaps for apparel),the number of CH2 segments between the amide sections should be increased, such as in nylon (6/12).
The second-highest-volume polyamide is nylon (6), which, as the numerical designation implies, is formed from a single monomer (see Figure 8.2c). A convenient source of the 6-carbon monomer is a ring compound that opens during the polymerization process to form the desired monomer. The ring that is used to form nylon (6) is called caprolactam.
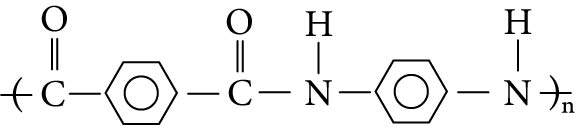
Figure 8.3 Aramid polymer repeating unit (Kevlar®).
This polymerization method is low in cost and creates a polymer with properties that are competitive with and similar to nylon (6/6).
Several copolymers, alloys, and blends have been developed with nylon as the principal component, and these have a wide range of physical properties, especially toughness and resistance to environmental conditions such as sustained high temperature and hot water. For applications requiring additional strength or toughness, there are nylons reinforced with short glass or carbon fibers or with minerals.
8.3.3. Aramids
A quite different polyamide material is illustrated in Figure 8.3. This material contains the amide groups with benzene rings between them. This highly aromatic polymer has been given a generic name, aramid, which seems to be a contraction of "aromatic polyamide." It is not generally called a nylon.
The properties of the aramids are unique among polyamides. The high aromatic content, especially when part of the backbone, stiffens and strengthens the material beyond any of the other nylons. When made into a fiber, the material is called Kevlar®, a DuPont trademark, and is widely used in making bulletproof vests and as a reinforcement in composites. The aramids are nonburning, solvent resistant, and very high melting. If the polymer is changed slightly so that the amide groups attach to a different carbon on the benzene ring (rather than straight across the ring), a molding plastic resin is created called Nomex®, a DuPont trademark. It is nearly as strong as Kevlar® and is more easily processed in standard molding equipment. It can also be used as a coating to strengthen and add flame retardance to many other materials, such as paper and cloth. Typical aramid products are shown in Photo 8.3.
.jpg)
Photo 8.3 Various aramid (Kevlar and Nomex) applications. Aramid helmet and Nomex clothing.