Osmosis
Osmosis
Osmosis, by definition, entails the movement of water through a selectively permeable membrane from an area of low solute concentration to an area of high solute concentration. For osmosis to occur, the membrane must be permeable to water but impermeable to the solute, and there must be different concentrations of solute between the two sides of the membrane. If the solutes cannot cross the membrane, then water molecules will naturally migrate from the side with lower solute concentration to the side with higher solute concentration until equilibrium is reached. Water will always migrate to establish equilibrium unless some other external force prevents it (i.e., osmotic pressure, but we’ll let the chemists address that!). Importantly, osmosis is a passive process, requiring no ATP expenditure for water movement.
In the body, water movement into or out of cells depends on the solute concentration (osmolarity) of both extracellular and intracellular fluids. When the extracellular fluid's solute concentration is lower than that of the cell, water flows into the cell, causing it to swell. To understand why cells, expand or shrink in different solutions, it's essential to differentiate between osmolarity and tonicity.
Osmolarity
Osmolarity quantifies the number of moles of particles per liter of solution, which is different than the more common term of molarity which measures the number of moles of molecules per liter of solution. Why the need for different units? Because the molarity of a solution is not always the same as the solution’s osmolarity. This is due to the nature of substances and how they behave differently in solution: for instance, when the ionic compound NaCl dissolves, it dissociates into Na+ and Cl- ions due to ionic bonding, effectively doubling the particle count. Therefore, a one molar solution of NaCl becomes a two osmolar solution (technically, around 1.8 osmolar due to incomplete dissociation). In contrast, glucose remains intact in water due to covalent bonding, so a one molar solution of glucose is also one osmolar.
The typical solute concentration in body fluids ranges from 285–295 milliosmoles per liter (often rounded to 300 for simplicity), denoted as mOsmoles/liter (mOsM), where the 'm' stands for 'milli' or one-thousandth of an osmole. Using osmolarity allows us to describe solutions with terms like 'isosmotic' (same concentration of particles in two solutions), 'hyperosmotic' (one solution more concentrated than the other), or 'hypoosmotic' (one solution less concentrated). These prefixes—'iso' meaning 'same,' 'hyper' meaning 'more,' and 'hypo' meaning 'less'—help us describe the solution's nature. Importantly, osmolarity considers all particles in the solution; thus, a liter of solution containing one mole of glucose and one mole of NaCl would be a three osmolar solution.
Tonicity
When discussing solutions and their impact on the body, tonicity emerges as a pivotal concept. The term 'tone' denotes the firmness or stretch of a tissue; hence 'tonicity' describes how a solution influences the firmness or stretching of a cell when immersed in it. This concept delves into the interaction between membranes and particles, helping to explain why cells respond differently to various solutions. The tonicity of a solution predicts the effect of the solution on the cell volume at equilibrium. Cells react to different solutions due to the behavior of particles in diffusion. Particles naturally diffuse from regions of high concentration to those of lower concentration until equilibrium is achieved. However, if the membrane isn't permeable to these particles, water will diffuse instead through aquaporins in the cell membrane to reach equilibrium. We call these non-permeable particles osmotically active particles. Thus, to reach equilibrium, water must move instead and if water exits a cell, the cell shrinks, and when it enters, the cell swells.
In an isotonic solution, where the concentrations of osmotically active particles are already balanced, the cell maintains its shape because there is no net movement of water or solutes across the membrane. Isotonic solutions contain the same concentration of osmotically active particles (non-permeable particles) as the cell. If the cell swells, the solution is termed hypotonic, whereas if it shrinks (crenates), the solution is hypertonic. When considering fluids and ions inside the cell (intracellular fluid) and outside the cell (extracellular fluid), equilibrium is typically reached through the movement of solutes (ions) or water, depending on membrane permeability. However, there are exceptions to this rule in biology. For instance, some solutes can enter the cell and subsequently disappear due to cellular metabolism.
Consider glucose, which can permeate healthy cell membranes. Upon entering the cell, glucose undergoes rapid metabolism, effectively vanishing from the extracellular fluid (ECF). This continual removal of glucose from the ECF sets up a concentration gradient that drives more glucose into the cell. With each glucose molecule transitioning from the ECF to the intracellular fluid (ICF), the ECF's concentration diminishes relative to the ICF's. Consequently, water flows into the cell to maintain equilibrium, resulting in cellular swelling. To clarify further, let's examine a scenario where a 300 mOsM solution of glucose—is considered isosmotic. However, when this solution is combined with the cell's contents, glucose begins moving into the cell along its concentration gradient. This might seem counterintuitive, as the concentrations appear equal. However, the 300 mOsM concentration within cells isn't solely due to glucose but rather a combination of ions like K+, Na+, and other things like proteins. Thus, concerning glucose, the gradient is from the ECF to the ICF.
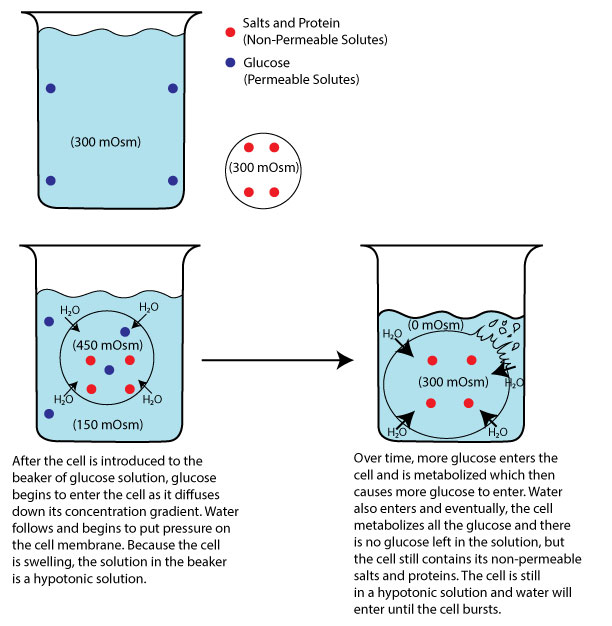
Image by JS F24
You can conceptualize osmolarity as dots per volume. In this simplistic analogy, if four dots represent 300 mOsM, the cell contains four dots (composed of K+, Na+, proteins, etc.), and the solution contains four dots (consisting solely of glucose). Consequently, both solutions are considered isosmotic, with four dots. Imagine that the four dots represent 300 mOsM. However, this term alone doesn't predict the outcome when they are mixed. Therefore, we rely on the concept of tonicity to understand the practical effect of such combinations. As each molecule of glucose moves into the cell, the outside concentration will begin to decrease from 300 to 299 to 298 etc. The inside osmolarity won’t necessarily increase because the glucose entering is being immediately metabolized, but the 300 (ICF) compared to the 298 (ECF) is not at equilibrium, and as glucose keeps moving that number will eventually drop to zero. Thus, water will start to move into the cell to try and equilibrate the two concentrations (ICF to ECF). Since the ICF is 300 and the ECF essentially zero, water will move into the cell trying to establish equilibrium. In this case, water would move into the cell so much that the cell membrane would burst. This scenario illustrates how a solution which is initially isosmotic with body fluids can act as a hypotonic solution due to the cell's metabolic activity.
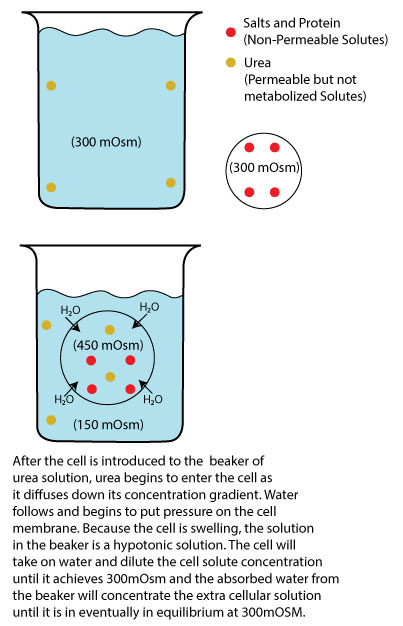
Image by JS F24
On the other hand, permeable solutes like urea that enter the cell but are not metabolized can also affect cell equilibrium. When a cell is placed in an isosmotic urea solution (300 mOsM), urea initially moves into the cell, reducing its concentration outside the cell while increasing the total solute concentration inside the cell. Even though the cell is also at 300 mOsM, remember that the 300 inside the cell is due to other solutes (K+, Na+, proteins, etc.), and since solutes behave independently of other solutes, urea will begin to move into the cell trying to reach its own equilibrium, say 150 mOsM (ICF) to 150 mOsM (ECF). As a result, the inside of the cell will become 450 mOsM (300 initial + the new150 from urea) and the outside will be at 150 mOsM. Since the concentrations are not at equilibrium, water will move into the cell to restore equilibrium, leading to cell swelling. In reality, this happens almost simultaneously, but by explaining as if it didn’t happen simultaneously, we can see that any isosmotic or hyperosmotic solution containing only permeable solutes will behave as a hypotonic solution to the cell. The end effect is a swollen cell (hypotonic) but with the same ending mOsm (450 (ICF) + 150 (ECF)) = 600 then 600/2 = 300 mOsM). We could also add a cell into a hyperosmotic solution of urea (600 mOsm) and see that it will always be hypotonic (cell swells) but end with a higher mOsm (600 (ICF) + 300 (ECF)) = 900 then 900/2 = 450 mOsM).
For this reason, osmolarity is not the best term to describe a solutions effect on a cell. A 300 mOsM solution of NaCl (0.9% saline) would be considered isosmotic but would have no effect on a cell shape because the NaCl compound is composed of non-penetrating solutes, thus it would also be considered isotonic. However, a 300 mOsM solution of glucose (5% dextrose), also considered isosmotic, would have drastic effects on cell swelling and would be considered hypotonic. *Note: while it is true that some Na+ can leak through cells, the Na/K ATPase pump removes the sodium at about the same rate, making Na+ “functionally” non permeable.
Understanding both terms of osmolarity and tonicity offers different perspectives on solution effects. Osmolarity compares solute concentrations between solutions or between a solution and the cell before equilibrium. Tonicity, however, describes the solution's effect on the cell and depends on the concentration of non-permeable solutes. While osmolarity disregards solute nature, tonicity considers it, focusing on non-permeable solutes.
The figure below shows what happens to red blood cells when they are placed into hypertonic, isotonic, or hypotonic solutions.
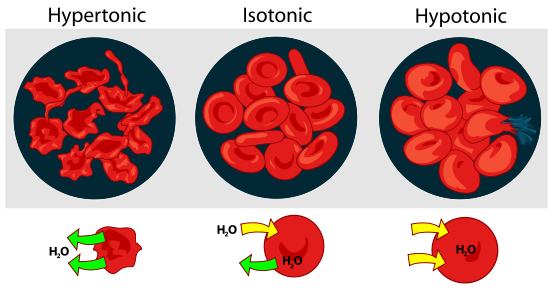
Osmotic Pressure on Blood CellsTitle: File: Osmotic pressure on blood cells diagram.svg; Author: LadyofHats; Site: https://commons.wikimedia.org/wiki/File:Osmotic_pressure_on_blood_cells_diagram.svg; License: Public Domain.
When placed in a hypertonic solution, red blood cells will shrink or crenate. When placed in an isotonic solution, there will be no change in volume, and when placed in a hypotonic solution, red blood cells will swell. If the concentration of the solution is great enough inside the cell, the cells will swell and even burst (lyse).
Here is a video to see what happens to a wilted plant when it is placed into a hypotonic solution.
To check understanding, complete the table below by filling in the missing column items with regard to osmolarity and tonicity. Use the terms iso, hypo, and hyper to complete the table.
| SOLUTION | OSMOLARITY | TONICITY |
|---|---|---|
| 0.9 % saline | ||
| 5% dextrose | ||
| 5% dextrose + 0.9% saline | ||
| 0.45% saline | ||
| 5% dextrose + 0.45% saline |
| SOLUTION | OSMOLARITY | TONICITY |
|---|---|---|
| 0.9% saline | Isosmotic | Isotonic |
| 5% dextrose | Isosmotic | Hypotonic |
| 5% dextrose + 0.9% saline | Hyperosmotic | Isotonic |
| 0.45% saline | Hypoosmotic | Hypotonic |
| 5% dextrose + 0.45% saline | Hyperosmotic | Hypotonic |
| Solution | Osmolarity | Tonicity |
Intravenous (IV) Solutions
In our previous discussions, we've primarily focused on placing cells into various solutions to elucidate the concept of osmolarity and tonicity. However, within the human body, the dynamic is somewhat reversed: instead of adding cells to solutions we are infusing a solution into the venous system (ECF) and allowing the solution to gradually surround cells throughout the body. In other words, cells in the body are naturally enveloped by solutions that maintain an equilibrium. Consequently, when intravenous (IV) solutions, with their own osmolarity, intermingle with the existing cellular surroundings, it prompts a consideration of how the shape of cells—tonicity—is affected.
When these IV solutions surround the cellular environment, they introduce a disruption to the delicate equilibrium within the cell. This disturbance could trigger osmosis, the movement of water across the cell membrane, in an attempt to equalize the concentration of solutes both inside and outside the cell.
Depending on the relative osmolarity of the IV solution compared to the cellular environment, different outcomes may arise. If the IV solution is hypotonic, meaning it has a lower concentration of solutes compared to the cell, water will rush into the cell, potentially causing it to swell or even burst in extreme cases. Conversely, if the IV solution is hypertonic, having a higher solute concentration, water will exit the cell, leading to cellular shrinkage or crenation.
It's crucial to carefully consider the tonicity of IV solutions in medical contexts, as their interaction with the cellular environment can have significant physiological implications. By understanding the interplay between osmolarity and tonicity, healthcare professionals can administer IV solutions tailored to the specific needs of the patient. An additional consideration about IV solutions is the variable of volume. We need to consider starting volumes and added volumes so that we can calculate ending osmolarity changes and the ending tonicity effect. Remember from the isosmotic example of urea above, even though the ending mOsM was 300, since the effect of the isomotic solution was hypotonic, the cell swelled. In an emergency, a practitioner may be able to get away with infusing a small volume of a hypotonic solution, because the larger body volume can buffer small changes in osmolarity, but the infusion of a large volume of hypotonic solution would be catastrophic.
Let's try a practice problem:
What would happen if we were to add a 2L IV solution containing 50 mosM of NaCl and 200 mOsM of urea to a person who has a total body water of 40 liters and an initial osmotic equilibrium of 300 mOsM. Assume that 2/3 water is found in the ICF and 1/3 in the ECF and then round to nearest whole number.
2L IV solution = 50 mosM NaCl/L = 100 mosm of NaCl; 2L IV solution = 200 mosM Urea/L = 400 mosm of Urea; 100 mosm of NaCl + 400 mosm of Urea = 500 milliosmoles of solute added.
From the previous section we also know that this solution is hyperosmotic (total concentration = 500 mosM) and also hypotonic (i.e., of the 500 mosM available, only 300 mosM are non-permeable). However, what will be the ending osmolarity of the system? Since osmolarity deals with solutes lets first determine the total solutes involved before any osmosis occurs (we will round to the nearest decimal):INITIAL CONDITIONS BEFORE IV INFUSION
| Fluid Compartments | Volume (L) | Osmolarity (mosM) | Solutes |
|---|---|---|---|
| Intra Cellular Fluid (ICF) | 27 (2/3 * 40) | 300 (given) | 8100 (300 *27) |
| Extra Cellular Fluid (ECF) | 13 (1/3 * 40) | 300 (given) | 3900 (300 * 13) |
| Total Body Water | 40 (given) | 300 (given) | 12,000 (8100 + 3900) |
BEFORE OSMOSIS BUT AFTER IV INFUSION
| Fluid Compartments | Volume (L) | Osmolarity (mosM) | Solutes |
|---|---|---|---|
| Intra Cellular Fluid (ICF) | 27 (given) | 300 (given) | 8100 (300 *27) |
| Extra Cellular Fluid (ECF) | 15 (13 + 2) | 293.3 (4400 / 15) | 4400 (3900 + 500 total solutes in IV) |
| Total Body Water | 42 (27 + 15) | 297.6 (12,500 / 42) | 12,500 (8100 + 4400) |
AFTER OSMOSIS
| Fluid Compartments | Volume (L) | Osmolarity (mosM) | Solutes |
|---|---|---|---|
| Intra Cellular Fluid (ICF) | 27.9 (8300 / 297.6) | 297.6 | 8300 (8100 + 200 half of permeable solutes) |
| Extra Cellular Fluid (ECF) | 14.1 (4200 / 297.6) | 297.6 | 4200 (4400 - 200) |
| Total Body Water | 42 | 297.6 | 12,500 (8300 + 4200) |
From this exercise, we observe that infusing 2 liters of an IV solution containing 50 mosM NaCl and 200 mosM urea would decrease the total body osmolarity by 2 mosM and add almost a liter to the ICF compartment, leading to cell swelling (hypotonicity). However, given that the total body water is 40 liters, the addition of 2 liters doesn’t cause significant cell swelling. This is primarily because although urea is permeable, it is not metabolized, thereby greatly reducing its hypotonic effect.
We highly recommend that you study this practice problem until it makes good sense to you. We will be doing more of these problems in lab and the better you understand now, the easier lab will go.